July CompuNet Updates
CompuNet Completes Cellavision DC-1 Rollout at Wayne HealthCare
CompuNet Clinical Laboratories launched the Cellavision DC-1 with Epic Citrix remote database at Wayne HealthCare on June 4th, 2025. The Cellavision automates and enhances hematology peripheral blood smear reviews using a digital microscope to take images of WBC, RBC, and platelets. Through an Epic Citrix Cellavision application, the technologist can review the blood smear and perform a differential on a computer screen rather than a traditional microscope.
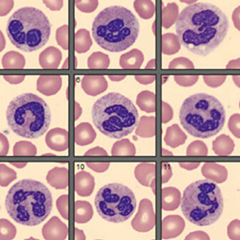
With this installation, all CompuNet Laboratory facilities are now equipped with this technology to allow shared viewing of images across all CompuNet laboratories to assist with problem cases and Pathology consultations. In addition, this technology will enhance turnaround time, provide educational and competency material for laboratory staff, improve ergonomics and standardize the quality of reporting.
For questions, reach out to Catherine Hoesl, MT(ASCP), system technical director of hematology and coagulation at cchoesl@compunetlab.com.
New Test Launch: Cystatin C with eGFR
CompuNet Clinical Laboratories is pleased to announce the launch of Cystatin C with eGFR testing beginning July 14, 2025. Cystatin C is a biomarker that can be used as an alternative to creatinine to estimate kidney glomerular filtration rate (eGFR). It may serve as a more reliable marker of renal function in situations where previous kidney function tests have provided unclear results or in individuals with higher muscle mass. This test offers clinicians an additional tool for more accurate assessment of kidney function, helping support improved diagnosis and management decisions for patients with or at risk for kidney disease.
Versiti Implements Low-Yield Platelet Strategy to Optimize Blood Supply
Effective July 14, 2025, Versiti Dayton Blood Center will update its thresholds for platelet donations to include low-yield platelets (2.6 – 2.9 x 10¹¹; current standard is 3.0 – 6.0 x 10¹¹).
The new testing and manufacturing process can occasionally produce platelet products that fall slightly below the current standard. However, analysis has shown that low-yield platelets are safe, effective, and do not increase the need for additional transfusions. This practice is already in place at several other blood centers nationwide.
Versiti has indicated that low-yield platelet products will account for less than 5% of all platelet donations. These units will be identified with a yellow sticker and may be used for any patient population. This change is part of an ongoing strategy to optimize the use of a constrained and critical resource.
Back to the July 2025 issue of Premier Pulse

